Describe Entropy in Your Own Words
With the teacher in the hallway the classroom descended into entropy. In your own words describe the evolutionary trend for increasing organismal complexity using examples from this lab to illustrate your answer.
Sue prevents her small apartment from falling into entropy by storing items in containers and on shelves.

. It is an extensive property of a thermodynamic system which means its value changes depending on the amount of matter that is present. What is water cycle in your own words. In the hydrological cycle we find several components that we will discuss together.
Explain the processes and the energy gains and losses involved in the changes of water between its 3 states. H X P X 0 l o g 2 P X 0 P X 1 l o g 2 P X 1 Lets take the word baseball from your example. Chemistry questions and answers.
G Describe in your own words using the Language of Enthalpy Entropy and Gibbs Free energy why a process might g Describe in your own words using the Language of Enthalpy Entropy and Gibbs Free energy why a process might be non-spontaneous at low temperature but spontaneous at High Temperature. It will contain an overview of what you have to say about these three topics. Search the internet and include an example of both besides the ones described in the.
Entropic interactions between objects are the inherent desire for disorder in the system. Define entropy in your own words and list the variables or conditions that you must consider when comparing the entropy of two substances or when trying to determine the relative change in entropy. In equations entropy is usually denoted by the letter S and has units of joules per kelvin JK 1 or kgm 2 s 2 K 1.
In information theory the entropy of a random variable is the average level of information surprise or uncertainty inherent. Be sure to describe how it relates to the states of matter at the submicroscopic level. Entropy is the degree of chaos or disorder in a system.
In this experiment we calculated the solubility of KNO 3 at different temperatures and used these values to determine the Ksp. It may be interpreted as a measure of the dispersal or distribution of matter andor energy in a system and it is often described as representing the disorder of the system. Entropy is the measure of the disorder of a system.
Enthalpic interactions are attractive and repulsive interactions between neighboring objects. N A certain property of a body expressed as a measurable quantity such that when there is no communication of heat the quantity remains constant but when heat enters or leaves the body the quantity increases or diminishes. State the second law of thermodynamics in words and equations and use it to predict.
We review their content and use your feedback to keep the quality high. X 1 if the word occurs in the sentence and X 0 otherwise. In your own words describe what Shannon entropy is.
11In your own words describe the relationship between state change and entropy. A campfire is an example of entropy. ΔH is change in enthalpy or energy in a system and TΔS is difference in entropy multiplied by temperature and entropy is the energy that is not used for work and accumulates as waste heat.
Answered 5 years ago. Explain in your own words the difference between a spontaneous reaction and one that occurs instantaneously. We can calculate the entropy of X using the formula below.
In endogenic reaction its non-spontaneous so it does require energy decrease entropy use dehydration and is anabolic. How can we use Chemical Thermodynamics to explain different processes involving entropy. Chapter Objectives Describe the scientific and economic obstacles to more widespread recycling of plastics.
Apr 12 2014. In your own words describe the behavior of the entropy of a substance as it changes phase from solid to liquid to gas. Entropy S is a state function that can be related to the number of microstates for a system the number of ways the system can be arranged and to the ratio of reversible heat to kelvin temperature.
The G was calculated from the equation G -RlnK. We see evidence that the universe tends toward highest entropy many places in our lives. Entropy always increases as.
In your own words describe entropy and enthalpy. Lets consider a binary random variable X which is associated with precisely one word. Describe the chemistry involved in this experiment in your own words and the significance of your results.
An anabolic pathway is a process in which complex molecules are built from simpler ones requiring free energy. In your own words describe what enthalpic and entropic interactions are. Who are the experts.
If a small amount h of heat enters the body when its temperature is t in the thermodynamic scale the entropy of the body is. Generally information entropy is the average amount of information conveyed by an event when considering all possible outcomes. Was energy transferred from the water to the chlorine gas or from the chlorine gas to the ice.
When comparing the relative. - 23669450 papichuloneedsanswer papichuloneedsanswer 16122021. What each part does.
In your own words write a few sentences that describe the difference between thermodynamic entropy and the second law of thermodynamics. Experts are tested by Chegg as specialists in their subject area. The various parts of a stroke engine basically work in unison in converting the thermal energy to mechanical energy.
Describe each part of a four stroke engine in your own words. Examples of Entropy in a sentence. Based on its fundamental definition explain why entropy is a measure of energy dispersion.
The difference between anabolic and catabolic pathways is the change in free energy as a result of the reaction. Up to 24 cash back Worksheet on Entropy 1. Then describe one way it can be used in bioinformatics.
Be sure to use evidence. It increase entropy use hydrolysis and is catabolic. Explain the concept of entropy in your own words.
The solid wood burns and becomes ash smoke and gases all of which spread energy outwards more easily than the solid fuel. Deduce the sign of Δ S for many chemical reactions by examining the physical state of the reactants and products. Write a paragraph that describes the difference between thermodynamic entropy and information entropy.
In your own words describe the difference between anabolic and catabolic pathways. Entropy is a measure of the energy dispersal in the system. Support your claim with evidence.
When then used the Ksp of each trial and calculated the G S and H. When the dictator died unexpectedly the country slid into entropy. The older Ted became the faster his body fell into entropy.

Factor That Influence The Entropy Of A System Teaching Chemistry Entropy Molar Mass

Laws Of Thermodynamics Pdf Teaching Chemistry Physics And Mathematics Thermodynamics

Chemistry Notes Spontaneity Entropy And Gibbs Free Energy Chemistry Notes Biochemistry Notes Entropy Chemistry

Space Flight Systems Website Undergoing Maintenance Glenn Research Center Thermodynamics Physics Books Work Physics

Entropy General Physics I Lecture Slides Docsity

Still Not Sure It Fits This Board Aesthetic Words Entropy Words

Entropy The Unseen Threat To Startups Entropy Geometry Tattoo Thermodynamics

Image Result For Art List Of Action Verbs Verbs List Richard Serra Serra

What Thermodynamics And Economy Have In Common Creative Fields Entropy Thermodynamics Physics
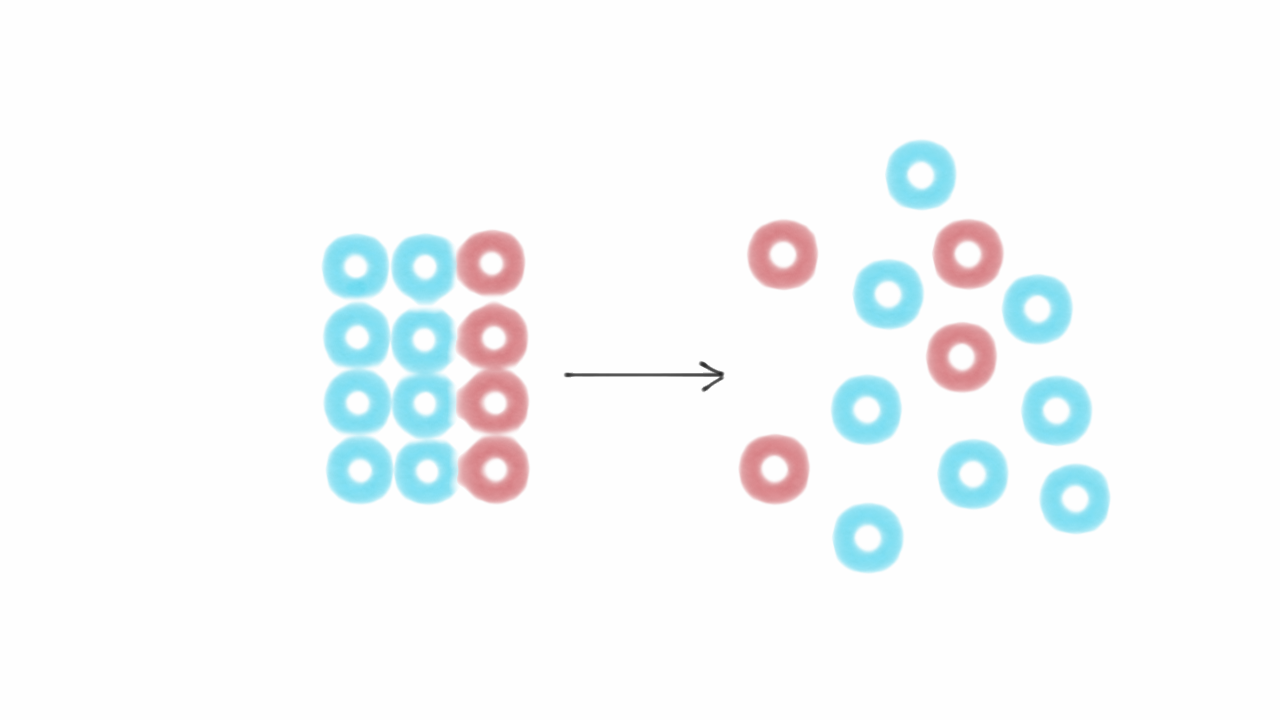
Entropy The Hidden Force That Complicates Life Farnam Street

Thermodynamics Is A Branch Of Physics Concerned With Heat And Temperature And Their Relation To Energy And Science Chemistry Thermodynamics Engineering Science

What Is Entropy Definition And Examples

Entropy God S Dice Game The Book Describes The Historical Evolution Of The Understanding Of Entropy Alongside The Entropy Dice Games Communication Theory

Define Entropy Data Science Machine Learning Data Scientist
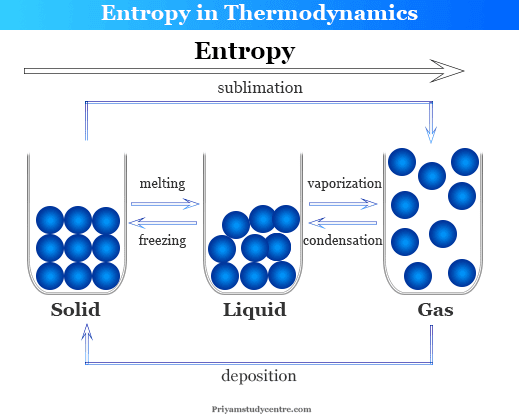
Entropy Change Definition Formula Equation Universe

Pin By Corneliu Dica On Thermodynamics Knowledge Management Theoretical Computer Science Entropy

Entropy Easy Science Easy Science Entropy Definition Entropy

Entropy In Chemistry Definition Law Video Lesson Transcript Study Com


Comments
Post a Comment